
First law of thermodynamics and law of conservation of energy the push force does work on the air, thereby, increasing its internal energy, which is shown, by the increase in temperature in the air. when the piston is rapidly pushed, the thermometer shows a temperature rise due to the increase of the internal energy of the air. It consists of a bicycle pump with a blocked outlet that allows the air temperature to be monitored. one such simple arrangement is shown in the figure. when we pump on the handle rapidly, it becomes hot due to mechanical work done on the gas, raising their by its internal energy. See Also : Second law of thermodynamics What is an example of the first law of thermodynamics?Ī bicycle pump provides a good example. Consequently, the body temperature or in other words internal energy is maintained by the food we eat.
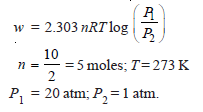
Work (W) done will result in a decrease in the internal energy of the body. We can apply the first law of thermodynamics: 1st law of thermodynamics formula Energy transforming processes that occur within an organism or named as metabolism. Energy is also needed for growth to make new cells and to replace old cells that have died. When they walk, run, or move heavy objects, work requires energy. When we pump on the handle rapidly,it becomes hot due to mechanical work done on the gas, rising thereby its internal energy.
First law of thermodynamics calculator plus#
“In any thermodynamic process, when heat Q is added to a system, this energy appears as an increase in the internal energy ΔU stored in the system plus the work W done by the system on its surroundings.”Ī bicycle pump provides a good example.


 0 kommentar(er)
0 kommentar(er)
